Many companies offer a complete solution to support your endoscopy suite, such as STERIS, helping you through every step of the processing cycle starting at bedside, through cleaning, to liquid chemical sterilization or high level disinfection, to compliant scope storage and transport solutions. Our products make it easy for you to quickly process your endoscopes, increasing throughput while maintaining the highest level of patient care and compliance to standards and guidelines.
Endoscope Point of Use Pre-Cleaning
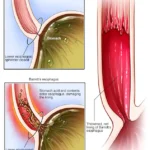
Endoscope cleaning begins immediately at the bedside and continues through transport with pre-cleaning solutions that prevent soils from drying while protecting devices from damage. Browse endoscope point of use treatment products, including solutions like PRE-KLENZ™ Soak Shield, which help loosen soils at the bedside and throughout transport.
Pre-cleaning kits, including enzymatic and non-enzymatic options, provide a ready-to-use solution for bedside cleaning. STERIS’s single-use cleaning adapters flush endoscopes at the bedside to begin the cleaning process. Select a pre-cleaning solution below to learn more and request a quote. STERIS endoscope point of use processing products help you meet evolving standards and ease the cleaning process.

- Bedside pre-cleaning products prevent drying of hard-to-clean soils on endoscopes at the point of use, making them easier to clean during processing.
Manual Cleaning
- One of the most important steps in endoscope reprocessing is manual cleaning with sponges, detergents, and brushes. Our portfolio of products is designed to break down soils and protect the integrity of delicate devices.

VERIFY® RESI-TEST™ Cleaning Indicators
- Quick cleaning verification results in 10 seconds
- Easy-to-read color change test results
- Conforms to ANSI/AAMI standards for cleaning verification tests1
High-Level Disinfection
High-level disinfection (HLD) can be achieved with a manual or automated process. A manual HLD process typically involves soaking a device for some time within a specific temperature and concentration. For most endoscopes, industry standards/guidelines recommend automated processes to provide a more efficient, consistent, and measurable outcome.

Automated Endoscope Reprocessors
An AER is often used for endoscope reprocessing. After cleaning, the automated process in an AER circulates the HLD solution around the exterior and through the device channels for a timed exposure period, followed by rinsing. STERIS AERs are available in either one-sided or pass-thru configurations. Both support best practices by allowing for unidirectional workflow.